

The first round of PCR amplifies the receptor locus and adds known sequences that act as priming sites for the second round, in which sequencing adaptors and indexes are incorporated. Multiplex PCR is appropriate for both DNA and RNA templates and generally consists of two rounds of PCR (after cDNA synthesis, if RNA is used as a template). The most common method of preparing sequencing libraries for immune profiling is multiplex PCR. Researchers often start with bulk analysis and move to single cells after selecting for binding affinity or other phenotypes of interest. However, analyzing single cells generally decreases the number of samples that can be analyzed. Since individual T cells or B cells express a single receptor sequence, single-cell analysis makes it possible to capture both the sequence and the pairing information. Thus, this pairing information is required for the identification of the actual TCR or BCR molecule in question. Functional TCRs are made up of paired alpha and beta chains, while BCRs have paired heavy and light chains.

Bulk analyses can sample many more sequences in a single experiment but do so at the cost of pairing information. Generally, analysis of immune repertoire diversity tends to rely on bulk approaches, while studies focused on identifying TCR or BCR sequences specific to a particular antigen use single-cell analysis. The choice to use bulk or single-cell analysis depends on the goal of your experiment. This is particularly important for antibody therapy or T-cell therapy applications. Full-length sequencing also makes it easier to directly clone and express those receptors identified in immune repertoire sequencing studies.
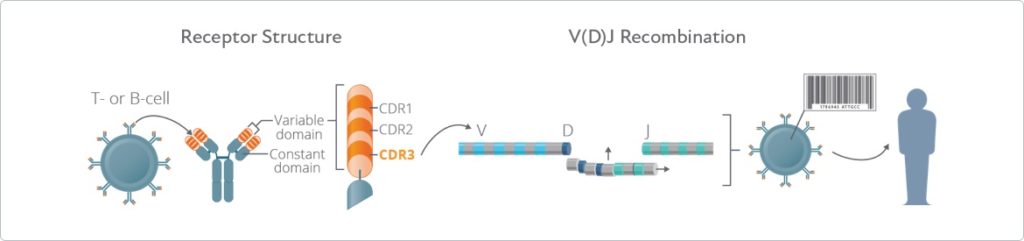
However, full-length sequencing will include additional regions of the receptor, including CDR1 and CDR2, which play roles in the affinity of antigen-receptor binding and/or downstream signaling. The CDR3 region contains the core site of interaction between antigen and receptor and is, therefore, the most variable, so this is not surprising. Most immune profiling experiments have focused on the CDR3 region of TCR and BCR sequences. Additionally, template choice can determine your options for NGS library prep (see below). DNA-based approaches, by contrast, do not identify receptor sequences in their translated forms, and will unavoidably yield many functionally irrelevant sequences. Thus, using RNA allows for a more comprehensive identification of unique receptor variants, including those that are present in a very small proportion of cells.Īdditionally, sequencing mRNA reveals the sequence of expressed receptors that have undergone splicing and post-transcriptional processing, and thus are more likely to yield functional proteins.

Because each cell has multiple copies of the TCR or BCR transcript, RNA-seq approaches are more sensitive. Using genomic DNA as a template can make it easier to determine the relative abundance of clonotypes, as each cell only has a single template. What factors should I consider when choosing an immune repertoire sequencing approach? DNA vs.
#Lossius a immune repertoire how to
Along with this added technology in the researcher's toolkit comes several key decision points on how to proceed with your studies. Although these methods have yielded valuable insights, the continued development of next-generation sequencing (NGS) has dramatically expanded the scale of repertoire profiling research. Traditionally, immune repertoire profiling has relied on cloning and Sanger sequencing or functional methods for identifying antigen-specificity (e.g., tetramer assays/stains). However, it is this very diversity of receptor sequences that can make it difficult to analyze them, and a myriad of research applications-from examining the mechanisms behind autoimmune diseases and cancers to developing models for immunotherapy-rely on being successful in this first step. Ultimately, this process yields cell populations with sufficient TCR and BCR diversity to recognize any peptide imaginable. To this end, the adaptive immune system has evolved a system for somatic diversification, commonly referred to as V(D)J recombination. Receptor-antigen interactions are specific, meaning a tremendous amount of diversity in TCRs and BCRs is required to recognize the wide assortment of antigens one might encounter. Adaptive immunity relies on the ability of the body to recognize and respond to a nearly endless number of molecules, a complex feat achieved through T-cell and B-cell receptors (TCRs and BCRs, respectively).


 0 kommentar(er)
0 kommentar(er)
